Difference between revisions of "Energy"
| Line 177: | Line 177: | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| Line 316: | Line 299: | ||
<br> | <br> | ||
| − | |||
=== Potential Energy – Kinetic energy changes during free fall === | === Potential Energy – Kinetic energy changes during free fall === | ||
Revision as of 16:12, 25 September 2012
Introduction
This is a resource document for teachers on the various topics of energy covered in high school. This contains resource and reference material for the teachers as well as classroom-based activities and discussions that the teacher can use to build conceptual clarity and understanding.
This resource contains sections on what does
energy mean, the relation between work done and energy, various forms
of mechanical energy and the units of measuring power. Further
conversion between various forms of energy is explored. The resource
also discusses renewable and non-renewable energy sources with a
detailed discussion on atominc energy as well. Energy flow in the
universe and eecosystem is also discussed to connect the living
ecosystem to the sources of energy, particularluy solar energy.
Energy storage and management are also discussed in this resource.
Where necessary, activities have been described in detail for the
teacher to use in the classroom. The resource also points to
additional web resources.
Energy is the basis of human
life. Every single aspect of human experience whether it be in the
external world or what we do or what is done to us can be adequately
described either as a transfer of energy in one form from one place
to another or the transformation of energy from one form to another.
What is the meaning of energy? How does one measure it? What are the
various forms in which energy manifests itself? How is energy
obtained and transformed from one form to the other? How can energy
be conserved? How do the production and utilization of energy in its
various forms affect our environment? What is the source of all
energy? What kind of energy flows and conversions take place in the
environment? These are some questions we will explore here.
Concept Map
Work, Energy and Power
We often use the terms work in ordinary conversation. Further, we also say that energy is needed to do work. We will explore the idea of work done, energy, power and energy conversions in this section.
Some of the key ideas to be covered in this
section are:
- Work has a physical meaning in relation to the force operating and this is linked to the concept of energy. Such work done can be measured in physical terms.
- What do we mean when we say an object has energy? We are introduced to the idea of kinetic and potential energy.
- Effectivesness of doing work is called power; work, energy and power have units of measure.
Concept flow
What does work done mean?
We often use the terms work in ordinary conversation. We say for example “This job requires a lot of work”. What does ‘work’ really mean here? Further, we also say that energy is needed to do work. We will explore the idea of work done, energy, power and energy conversions in this section.
Work always involves some opposing forces. What
do we mean by this? If we lift a box from the ground level and place
it on a high shelf, we feel tired after the job is completed; we feel
that we have done some work. How is this done? Gravity pulls the
box and hence we are doing work against this gravitational force.
This is true for any type of work.
Suppose that instead of lifting the box, we push
it across a rough floor. In this case, we are not working against
the gravitational force-the box is at the same height throughout the
movement. Instead, we are now working against the frictional force
that exists between the moving the box and the floor.
In physical terms, work done is
directly proportional to both the applied force and the distance
through which the force acts.
How do we measure work here?
We know that there is some cause, a force that
results in a change in state of an object. When such a change in
state occurs, it often results in a change in energy of the object.
Is this waiter carrying a tray doing work?
When force acts over a period of time, there is an
impulse I = F x t = m(v – mu) is the change in momentum of the
body. Let us now look at what happens when force acts on an object
over a distance along the direction of the force. In we say work is
done by a force if the force acts on an object over a distance and we
say that the work done W = Force F x distance travelled along the
direction of the force.
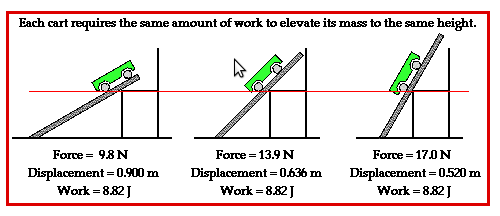 Notice
that the work done is the same in all the three cases above though
the force applied is different. Greater the angle of inclination,
greater is the force needed.
Notice
that the work done is the same in all the three cases above though
the force applied is different. Greater the angle of inclination,
greater is the force needed.
Thus W = F x d if the distance travelled d is in
the same direction as the force. If the direction in which the object
is moving is at angle θ then the distance travelled in the direction
of the force would be d Cos θ and the work done would be W = f x d
Cosθ.
 If
the force is acting perpendicular to the direction of the work done,
there is no work done. If you are carrying a heavy bag in your hand
and walking, though you may feel tired, no work is done in the
physical sense, because there is no energy change that has occurred
for the bag.
If
the force is acting perpendicular to the direction of the work done,
there is no work done. If you are carrying a heavy bag in your hand
and walking, though you may feel tired, no work is done in the
physical sense, because there is no energy change that has occurred
for the bag.
Work done, is defined as a particular form of
product of two vectors – force and displacement. Such a product is
called the scalar product and the resulting quantity is a scalar. In
the context of work done, this is easy to understand. You don’t do
work in a particular direction – you just do work.
The unit of work done is joules. 1 J of work is
said to be done when a force of 1 N causes a displacement of 1 m.
When work is done, it is done over a certain time.
The rate of doing work is defined as the power. Power is defined as
(work done)/ time taken.
Power = work done/ time = force x displacement/
time = force x velocity
Its unit is Watt.
Energy, types of energy-Kinetic and potential
When an object is moved against a force, work is done and energy is spent in the process. Thus we say “A person must have a lot of energy to do a hard day’s work”. In fact one way to define energy is: Energy is the capacity to do work.
The word ‘energy’ is derived from the Greek
energia'''-en means '''‘in’'''
and ergon''', means
'''work.
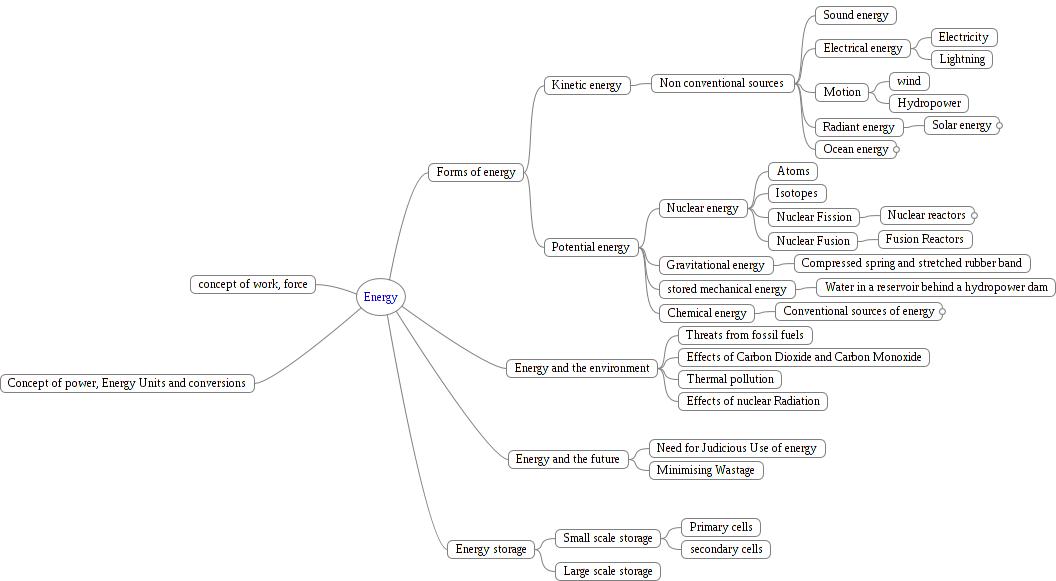
Energy is defined for an object in a particular state. When work has been done on an object, its energy changes. At a very basic level, there are two forms of energy – kinetic energy and potential energy.
For example, a block is lying at rest on a table.
It is pushed and it acquires a uniform velocity. Now the block has
acquired some energy (kinetic energy, as we will define shortly).
While the cause of the change in the state was a force (the push), it
has resulted in the body acquiring a change in energy.
We can see that there are two ways of describing
this (and for that matter, any) process. One is to study the cause
(the force) and the other is to examine the change in energy.
Understanding Kinetic Energy
If an object is moved from rest to a uniform
velocity, horizontally, it has acquired kinetic energy. Conversely,
if an object is moving with a velocity ‘v’ and has to be stopped,
work needs to be done. Let us understand this mathematically.
Let us say an object of mass ‘m’ is moving
with a velocity ‘v’ and is brought to rest by a retarding force
over a distance ‘s’
The
acceleration = v2/ 2s
The force required = m x a
= m x v2/
2s
Work done =
force x displacement
= (m x v2/
2s) x s
= ½ m v2
This is the expression of the kinetic energy that
the object had.
If this object is a car and it was brought to rest
by braking, the kinetic energy was lost as heat energy due to
friction (braking) between the road and the car tires. The total
energy remains conserved; it merely moves from one form to another.
All energy is due to
motion and is kinetic energy. The energy that an object possesses
by virtue of its motion is Kinetic energy.
 Understanding
Potential Energy
Understanding
Potential Energy
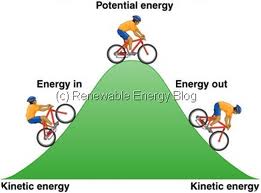
If an object is lifted from the ground to a
certain height, work has been done in moving it and this is stored in
the object as potential energy. If the object is dropped, it will
fall to the ground with a velocity and will acquire kinetic energy.
- Potential energy can be more usefully understood and described as potential for energy. When a body has potential energy, it has the capacity to do work. When a spring is compressed, work has been done on it. If it is released, the spring can do work. The potential (for) energy that it has allows the spring to do work.
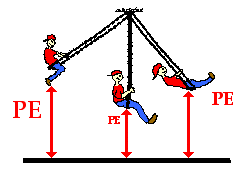 It is also useful to think of potential energy in terms of change in energy level with respect to a zero. The surface of the earth has been arbitrarily assumed to be at zero potential energy.
It is also useful to think of potential energy in terms of change in energy level with respect to a zero. The surface of the earth has been arbitrarily assumed to be at zero potential energy.- The potential for energy is with respect to a zero defined for a system. The potential energy is, therefore, always to be referred to in terms of a system. The potential energy of the object-earth system was changed when it was lifted to a height ‘h’.
If an object of mass ‘m’ is raised to a height ‘h’, work has been done.
Work done = m x g xh
= mgh
This is stored in the object as potential energy
and when the object falls, gets converted into kinetic energy. In
this particular case, the potential energy is referred to as
gravitational potential energy. An object can also possess potential
energy if it is put under strain. Then it has energy stored in it
because of the work done to bring it in that condition. In a time
piece, the main spring, for example, once wound keeps unwinding and
driving the clockwork mechanism for many hours. Here the coiled
spring has energy stored in it because of the work done on it while
winding.
Potential energy is the potential for energy that is built in an
object. The energy that an object possesses when it is lifted to a
height or it is put under strain is potential energy.
Activity 1 (Self-evaluation for student)
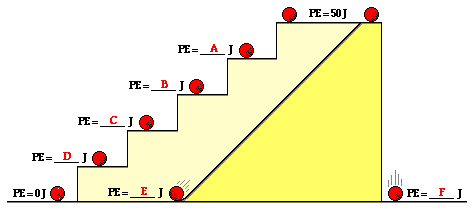 Knowing
that the PE at the top of the stairs is 50 J, what is the potential
energy at the various positions?
Knowing
that the PE at the top of the stairs is 50 J, what is the potential
energy at the various positions?
Potential Energy – Kinetic energy changes during free fall
Conservation of energy
The key idea here is that the total energy of the system is conserved. Potential energy can be converted into kinetic energy and vice versa. But the total energy remains unchanged.
In more general terms, the law of conservation of
energy states that energy can neither be created nor destroyed; but
can be transformed from one form to another.
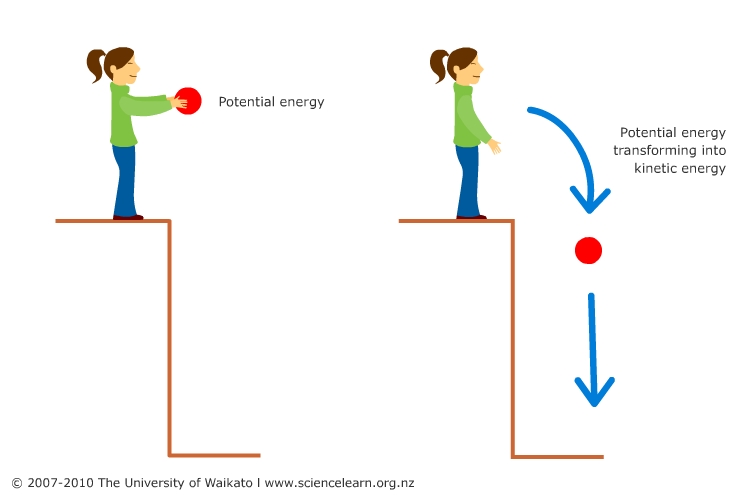
Activity : Conservation of mechanical energy in a pendulum
Objectives:
- To study the motion of a simple pendulum
- To observe the different factors on which the motion of a simple pendulum depends
- Develop an understanding that gravity is a conservative force
Method:
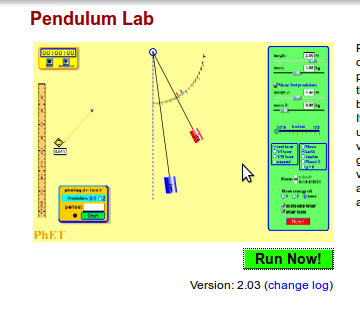 Use
the PhET simulation Pendulum Lab.
Use
the PhET simulation Pendulum Lab.
For this we will need to open an application
called PhET on the computer. You can find PhET under Applications>
Education> Science. PhET is an educational resource that contains
computer demonstrations of experiments and activities. When we click
on Play with sims – it will open simulations in various subjects.
We will click on Physics and scroll down to the simulation on
Pendulum Lab.
When we want to open a simulation, we click on
the green rectangle which says “Run Now”.
Running the simulation
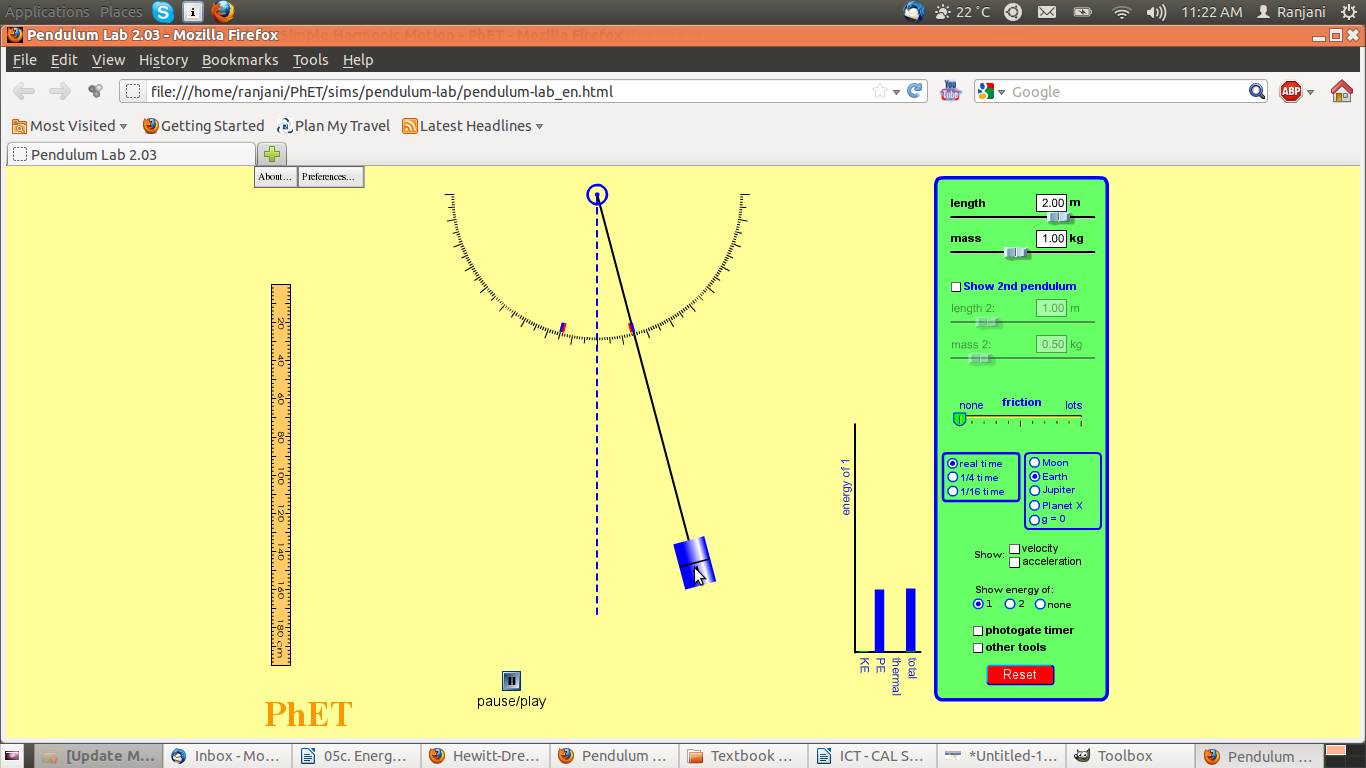
Screenshot #1
- Notice where the pendulum is – is it higher, lower or at the same level as the central position?
- Notice the graph – what are the two variables on the bar chart?
- What do you think will happen to the pendulum next?
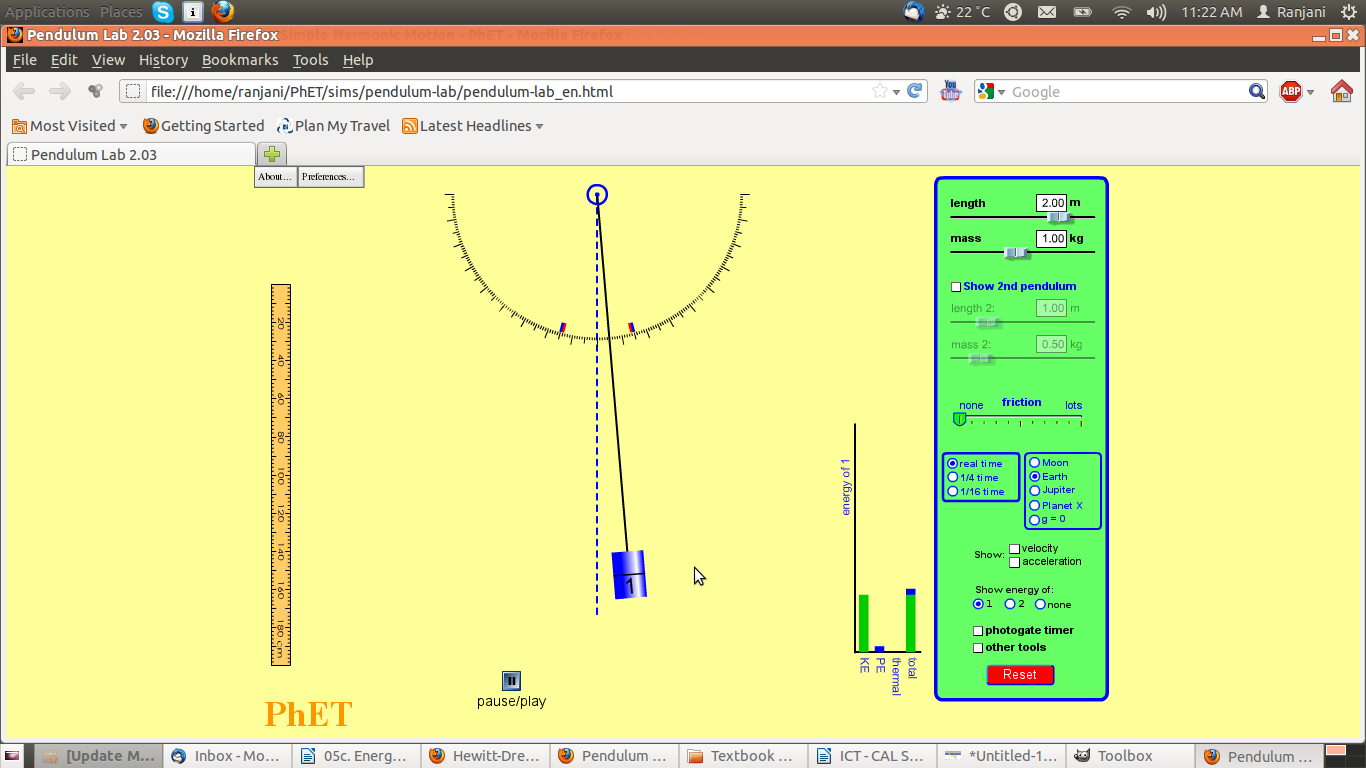
Screenshot #2
- Notice where the pendulum is – has it moved? What can you say about its movement?
- Notice the graph – what are the variables on the bar chart?
- What are the values of PE and KE as compared to total energy?
Screenshot #3
- Notice where the pendulum is – has it moved? Is it higher or lower than the central position?
- Did you notice anything about the speed of the bob as it moves from one extreme position to another?
- Notice the graph – what are the variables on the bar chart?
- What has happened to the values of the KE and PE as compared to total energy?
- What do you think is happening? Is this what you will think will happen when you try this experiment? Why? Why not? What is different?
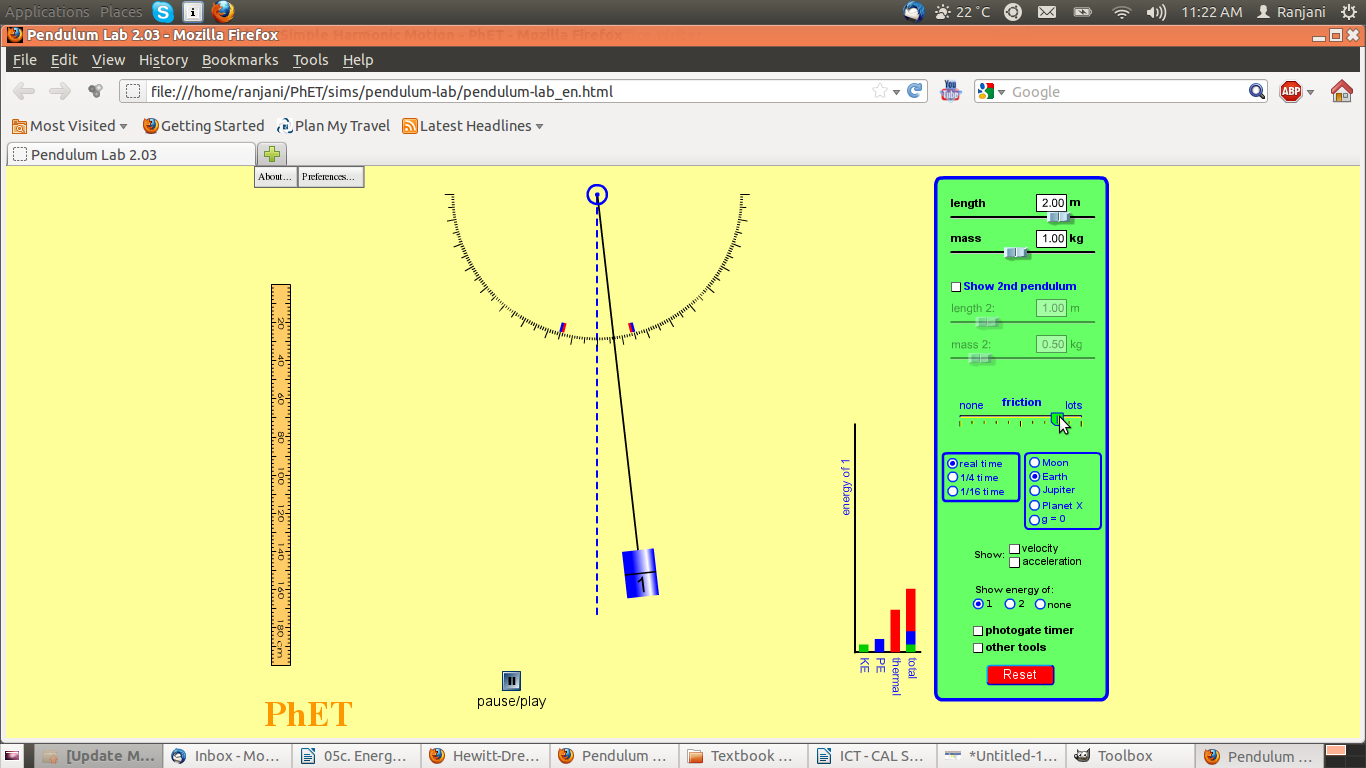
Screenshot #4
Questions:
- Notice where the pendulum is. This extreme position to the right is at a different height than before. Why? What role does friction play and where does it come from
- Look at the graph – what are the variables in the bar chart? Where has the thermal energy come from?
- What do you expect will happen to the simple pendulum?
Mechanics of the simple pendulum
The motion of a pendulum is a classic example of mechanical energy conservation. A pendulum moves it sweeps out a circular arc, moving back and forth in a periodic fashion. Neglecting air resistance (which would indeed be small for an aerodynamically shaped bob), there are only two forces acting upon the pendulum bob. One force is gravity. The force of gravity acts in a downward direction and does work upon the pendulum bob. However, gravity is an internal force (or conservative force) and thus does not serve to change the total amount of mechanical energy of the bob. The other force acting upon the bob is the force of tension. Tension is an external force and if it did do work upon the pendulum bob it would indeed serve to change the total mechanical energy of the bob. However, the force of tension does not do work since it always acts in a direction perpendicular to the motion of the bob. At all points in the trajectory of the pendulum bob, the angle between the force of tension and its direction of motion is 90 degrees. Thus, the force of tension does not do work upon the bob.
Since there are no external forces doing work, the
total mechanical energy of the pendulum bob is conserved.
Power, energy units and conversions
One important aspect of the processes producing or using or/and converting energy from one form to another is the rate at which this is done. For example, two persons perform equal amounts of work by lifting identical boxes from the ground level and keeping them on a shelf .One of them does this rapidly while the other does it slowly. Although the total work done by each person is the same, the two persons work at different power levels. The faster working person converts his body’s chemical energy into work at a more rapid rate than the slowly working person
Power is the rate at which work is done or energy
is used or supplied and may therefore be calculated by dividing the
work done (or energy used or supplied) in the process by the time
taken by the process. Work
is done over a certain time. Rate of doing work is power. Power is
different even if energy is the same.
Energy or work is measured in Joules (J) and time
is measured is seconds (s) and so the unit of power is the joule
per second (J/s), This unit is given the special name WATT (W)
where
1watt =1 J/s
1000watt =1
kilowatt=1kw
1000,000 watt =1megawatt=1MW
1,000,000,000watt =1gigawatt=1GW
1,000,000,000,000watt =1terawatt=TW
A commercial unit energy that we often hear about
on out electricity bills is the kilowatt hours (kwh).
1kilowatt hour is the energy used or supplied when 1kw power is used
or supplied for one hour. 1kwh is equal to 3.6 million joules.
Energy Units and conversions
The basic unit for the measurement of energy in the metric system is the joule, but there are also other units in common usage. The kilowatt hour is usually used to describe electrical energy. The calorie which is defined as the amount of heat energy required to raise the temperature of 1g of water through 1degree Celsius, is the unit primarily used to measure heat and also to describe the energy content of food stuff.
Energy in the world
The transformations of energy from one form to another and the efficiency of the transformation processes are studied in Physics, Chemistry and Biology. While so far we have discussed potential energy and kinetic energy (expressed as thermal energy in molecules), we see around us energy in various forms such as electrical energy, solar energy tidal energy hydro energy, geothermal energy and so on. All these forms of energy fall under the two categories of energy kinetic and potential. Potential energy is the stored energy and the energy of position and can be understood as potential for energy. Chemical energy, nuclear energy, stored mechanical energy and gravitational energy are all forms of potential energy.
In this section, we will explore the following:
- Matter contains internal energy caused due to molecular motion
- Energy can be conveted from one form into another
Energy in matter
The molecules in every bit of matter solid, liquid or gas are in a continual state of motion. This random motion of molecules(or atoms) constitute an internal kinetic energy or thermal energy that an object possesses even though the object as a whole may not be in motion. Thermal energy is thus manifestation of the motion of the molecules of a substance. A change in the thermal energy of an object can be brought about by supplying heat to the object. For example, by repeatedly hitting a block of metal with a hammer, its atoms are caused to move rapidly, thereby raising the thermal energy of the metal block which as a result becomes hot.
The primary source of all energy on the Earth is
the Sun, though there are some small amounts of energy that come from
the Earth's interior as well as cosmic radiation. Tidal energy is
also caused by the gravitational pull of the Earth. Powered by the
Sun, energy constantly flows through the Earth's surface and
environment.
Laws governing the energy of the universe
Studying the flow of energy and understanding the processes in the world is called thermodynamics. This branch of science involved asking questions about how processes happen in the world around us. For example, why are certain processes irrevesible? What makes a chemical reaction takes place? When does heat flow from one object to another?
Exploration of these concepts resulted in the
formulation of two laws:
- The first law of thermodynamics states that the total energy of a system and its surroundings remains constant. In other words, the energy of the universe is constant. This law implies that energy is conserved and the energy content of the system takes two forms - work and heat.
- The second law of thermodynamics states that the entropy of the universe is always increasing. This can be summarized by stating that the universe always moves in a direction of increasing randomness.
Some processes of energy conversion
We have seen that energy occurs in various forms. These different forms of energy can be converted from one form to another.
- For example, when we switch on an electric light firstly, we can see the transfer of electrical energy from the power plant to our home, and then the conversion of electric energy into heat energy, part of it into visible light. This light energy is not destroyed but it is absorbed by the walls, ceiling and floor and other objects, finally to be converted into heat. Electric energy → heat energy+ light energy
- In a power plant, chemical energy stored in fossil fuels such as coal, oil or gas is converted into heat energy in the boiler by combustion. This heat energy changes water from liquid state to steam. This heat energy of steam is converted in part, into mechanical energy in the steam turbine. This mechanical energy is then converted into electrical energy in the generator. From the generator it is transferred by the electric cables to various points where it can be used for further transfer to homes and industries etc., Chemical energy → heat energy → steam energy → mechanical energy electrical energy → light energy+ heat energy+ mechanical energy
- In the running of a car the chemical energy hidden in the explosive mixture of petrol vapour and air is converts by the spark into heat energy. The heat energy, in turn is converted in part, into mechanical energy of motion of the pistons in the cylinders. The mechanical energy of the pistons is transferred to the drive shaft and from there to the wheels to move the car. Chemical energy → heat energy → mechanical energy
- All biological processes throughout the domain of living things can also be shown to be energy conversion processes. The digestion of food is a combination of rather complicated processes but what it amounts to is the transformation of chemical energy locked in the food into heat energy to keep the body warm, and into mechanical energy to enable the body to do work by moving its various parts or itself as a whole besides synthesizing some compounds. There is also some conversion into electrical energy to establish communication between various parts of the body through the nervous system.
- Chemical energy → heat energy + mechanical energy + electrical energy In all the above examples energy is converted from one to another, but the total energy in any energy conservation process always remains constant; that is energy can neither be created nor destroyed. This is the law of conservation of energy.
Biological energy flow
We saw earlier that energy flows constantly through the Earth and its environment. Plants fix the solar radiation into carbohydrates and form the basis of much of the energy flow in the world. Either through the food chain or through the accumulation as fossil fuels, this accounts for the bulk of the energy in the world.
Concept Flow
- Energy flows are essential to life processes and we need to study biological energy flows also to understand flow of energy.
- We cannot discuss energy without discussing the connection with food and how energy flows through living organisms through food. This flow of energy through living organisms is called a food chain.
Energy flow in an ecosystem
An ecosystem is a community where living and non-living things interact. There are two main processes in an ecosystem – energy flow and nutrient flow. The energy flow in an ecosystem happens through the food chain.
Activity: Energy flow through a food chain
Objectives
- To understand the way energy flows in an ecosystem
- To explore the connections between different cycles and processes in an ecosystem
Method
Watch the following videos on food chain
- Food chain in Africa [[1]]
- Interactions in an ecosystem [[2]]
- Description of a food chain and web [[3]]
- Interactions and energy flow in an ecosystem [[4]]
Questions:
- When we say food web, what comes to your mind? Why do you think it is called food web?
- What are the implications of laws of thermodynamics on how much energy is transferred in a food chain?
- Can you think why there are few consumers and large number of producers? What happens when a consumer eats a producer?
- In the local ecosystem, provide examples of each of these categories in your area – producer, consumer and decomposer?
- For this web to work properly, what is needed?
- What is energy flow? How does it flow – from small organisms to large or large organisms to small? Why do you think so? What elements do you observe in the ecosystem that give you this idea?
- Is the tiger 'bad' because it ate the goats? Why is the tiger eating the goat?
Methods of energy flow
The laws of thermodynamics we saw earlier govern the processes in a food chain also. For example, the first law tells us that an organism can only use the energy it receieves whereas the second law tells us that not all of the energy received by an organism can be used – some of it will be lost as heat. At each level of the food chain, the amount of energy that gets transferred to the next trophic level is only a portion of the energy present in the lower level. This fraction varies widely across ecosystems. When finally organisms die and decay they pass the materials of life in simple forms to other organisms (nutrient flow). This energy flow is not cyclic – continuously less and less energy is available. So then, how do ecosystems continue? They depend on an external source of energy called the Sun. If the Sun's energy is not available in usable form, life on Earth may not be possible any longer.
Conventional sources of energy
We saw how the solar energy is responsible for producing organic compounds that sustain energy flow in the living world. Availability of energy resources and harnessing them for multiple applications have concerned the human society for centuties. Advances in technology and society have always been linked to the access of and use of natural energy resources. In this section, we will look at some of the major sources of energy that are used by human beings in our endeavours.
Concept flow
Some key ideas we will explore in this section are:
- Solar enerrgy is the source of almost all energy on the Earth
- Fossil fuels are exhaustible and needs thousands of years to form.
- Biomass energy which comprises parts of living things is also a source of energy.
Forest
Firewood has been the major source of energy during most of man’s history and it continued to remain the most important fuel until the middle of nineteenth century. Firewood is obtained from the forests and is primarily used for heating and cooking. The other fuel which has been traditionally used here is animal dung cakes. The animal dung mainly consists of undigested plant material which on drying, gives a product that readily burns. One of the disadvantages of both firewood and animal dung cakes as fuels is that they give a lot of smoke on burning.
With the industrial revolution in Europe, people
learned to transform the energy from coal to mechanical energy in
machines. This led to increased demand for energy. The discovery of
coal, followed by oil and natural gas fulfilled these demands to a
large extent and these fuels since then have been the primary sources
of world’s energy.
Fossil fuels
All these fuels - coals, oil and natural gas- are derived from the slow decay of living organisms such as trees, algae and small marine animals for millions of years and are therefore known as fossil fuels. The fossil fuels are being consumed at an appreciable rate. Although their new deposits continue to be discovered, the world reserves of these fuels are limited. Further, these energy sources take millions of years to form and therefore fossil fuels are also known as non-renewable sources of energy. These fuels once exhausted cannot be replaced quickly when exhausted. Hence these are also called as exhaustible resources.
Formation of fossil fuels
Coal
 Fossil
fuels-coal, oil and natural gas-are the result of decomposition of
living matter. Coal is obtained from dead plant matter which consists
primarily of carbon, hydrogen and oxygen.
Fossil
fuels-coal, oil and natural gas-are the result of decomposition of
living matter. Coal is obtained from dead plant matter which consists
primarily of carbon, hydrogen and oxygen.
 On
dry land, this matter rots away by bacterial action in presence of
atmospheric oxygen to form carbon dioxide and water. But in swampy
locations, the dead plant matter is covered with water and is,
therefore, protected from the oxidising action of air. Instead, it
is attacked by bacteria which do not require free oxygen in order to
live. In the process oxygen and hydrogen of the dead plant matter
gradually escape and the residue, therefore, becomes richer and
richer in carbon. The end product of the bacterial action is a
soggy, carbon-rich substance called peat.
On
dry land, this matter rots away by bacterial action in presence of
atmospheric oxygen to form carbon dioxide and water. But in swampy
locations, the dead plant matter is covered with water and is,
therefore, protected from the oxidising action of air. Instead, it
is attacked by bacteria which do not require free oxygen in order to
live. In the process oxygen and hydrogen of the dead plant matter
gradually escape and the residue, therefore, becomes richer and
richer in carbon. The end product of the bacterial action is a
soggy, carbon-rich substance called peat.
 Over
long periods of time the peat is covered with sand, silt and clay.
As peat gets compressed and heated further due to geological changes,
more gases are forced out and therefore the proportion of carbon
continues to increase. In this way, peat is gradually converted into
various forms of coal such as lignite, bituminous coal and
anthracite.
Over
long periods of time the peat is covered with sand, silt and clay.
As peat gets compressed and heated further due to geological changes,
more gases are forced out and therefore the proportion of carbon
continues to increase. In this way, peat is gradually converted into
various forms of coal such as lignite, bituminous coal and
anthracite.
Petroleum and natural gas
In contrast to coal, the raw material in the formation of oil and natural gas consists mainly of marine organisms, mostly plants that grow near the surface of the sea. When these organisms die and accumulate in basins, where the water is stagnant, they are also protected from atmospheric oxidation. The dead marine matter is decomposed by anaerobic bacteria. Oxygen, nitrogen and other elements escape leaving mainly compounds of carbon and hydrogen called hydrocarbons. The accumulating covering layer of sediments provides heat and pressure that convert the hydrocarbon material into droplets of liquid oil and bubbles of natural gas. As more sedimentary deposits are laid down over periods of time, the pressure increases and the oil and gas are forced into nearby porous sand or sandstone. Gradually the oil and gas migrate upward through the sand and they then either escape to the surface or are trapped beneath layers of clay stone. This migration process separates the oil from underground water because water molecule readily adhere to sand whereas oil molecules do not. Thus the oil tends to collect in the pore spaces of sandy rocks beneath roof rocks with natural gases on the top.
Biomass Energy
Fossil fuels are derived from plants, trees and animals that lives millions of years ago. It took the remains of these organisms millions of years of burial under tremendous pressure and the internal heat to turn into coal, oil, or gas that we use as fuel today. We cannot get fossil fuels from the plant and animal waste that we produce today. But they, too form a substantial source of energy in the form of biomass. Biomass means the waste material and dead parts of animals that are living today. It includes garbage, industrial waste, crop residue, sewage and plant waste such as dead leaves and wood. These wastes can be both wet and dry. Wet wastes are in the form of animal excreta or domestic and industrial residues. Dry wastes refers to leaves, wood, paper, straw, fruit skin and others. There are two ways of using biomass as a source of energy. One is to burn the dry biomass directly to produce heat and generate steam. Another method is to convert this biomass into gaseous fuels called biogas by fermentation.
The raw material used for the production of biogas
is cow dung mixed with water which is taken in an insulated,
air-tight container called digester. In the digester, bacteria break
the raw material into simpler chemicals by a process known as
anaerobic decomposition. Other bacteria then convert the chemicals
into a biogas for fuel. The gas consists of mainly methane and is
drawn out through a gas outlet pipe.
Wet wastes from household and industries too can
be used to produce methane gas. Wastes may be dumped in deep pits.
Wells are then drilled down into the waste. A pipeline is then
drilled down into the waste. A pipeline is then to recover the gas
produced by the natural decomposition of the material.
From fossil fuel to electricity
The most convenient usable form of energy is electricity. In all the sources of energy above, the heat generated from the combustion of fuel is used to boil water to produce steam which powers a turbine. This mechanical energy of turbine is converted into electricity in generators. These stages involve various losses and therefore the overall efficiency of these plants is never more than 40 percent. It is, however, possible to cut short the above energy conversion stages and convert heat from the combustion of fuels directly into electricity using a magneto-hydro dynamic generator, popularly known as MHD generator, which works on the basic phenomenon of electromagnetic induction. Another method of increasing the efficiency of power generation is also through the use of combined cycle power plants.
Processing of coal and petroleum
Coal, which is essentially pure carbon, is chiefly used as a combustion fuel. The reaction of carbon with atmospheric oxygen to produce carbon dioxide is an exothermic reaction that releases about 7,840 kilocalories/kg of carbon and this reaction is responsible for the heat energy derived from burning coal. Burning of coal produces large quantities of fly ash and noxious gases such as a sulphur dioxide and related compounds which cause atmospheric pollution. Coal is therefore converted into a cleaner fuel, coke, by heating crushed coal to high temperatures in the absence of air. Coal can also be converted into liquid and gaseous fuels which can partially replace the fuels derived from petroleum.
Unlike coal and natural gas which can be used
directly as fuels without processing, petroleum or crude oil is not
directly usable. The name ‘petroleum’ is derived from the Latin
words petra meaning ‘rock’ and oleum
meaning ‘oil’. Therefore, it means rock oil, to distinguish it
from animal or vegetable oils. Petroleum, also often called crude
oil, is a mixture of hundreds of hydrocarbon compounds together with
small amounts of compounds of other elements. The exact composition
of crude depends upon many factors such as its age and the types of
organisms from which it is formed. So, every deposit of crude oil
is a unique mixture whose exact composition differs even from
deposits separated from it vertically or horizontally by a few metres
of rock. Natural gas is normally associated with crude oil. It is a
mixture of gaseous hydrocarbons, mainly methane and ethane. The
non-hydrocarbon compounds present in crude oil are mainly compounds
of sulphur, nitrogen and oxygen. Other elements present in very
small amounts include vanadium, nickel, chlorine, arsenic and lead.
Detection of Oil
The method generally used for locating oil deposits is the seismic survey. Shock waves generated by surface explosive charges travel through rock layers and are reflected back by various geological structures and possible locations where oil might be trapped can be found. To find whether oil is really present and whether it can be economically extracted, it is necessary to drill a well.
Extraction and refining of Oil
Once the oil has been found by drilling the well, the next step is to operate the well; that is, to raise the oil to the surface. After extraction, the oil is usually transported to a refinery through pipelines. From offshore platforms, the oil is sometimes transported to the shore in large tankers. The natural gas produced in the process is also transported by large pipelines.
Crude oil is processed in a refinery by fractional
distillation. This process involves heating the crude oil in a tall
tower so that various components are distilled out of it and can be
trapped at various levels in the tower. In this process the use is
made of the fact that the different hydrocarbon compounds in the
crude have different boiling points and hence can be separated at its
boiling point. The lightest compounds such as gases which have low
boiling points rise to the top and the heavier oils with higher
boiling points are collected lower down.
The various fractions may then be further
processed by cracking or refining, both of which involve the use of
catalysts-substances which facilitate the chemical reactions without
themselves undergoing any change. Catalytic cracking, often called
cat-cracking, is a means of breaking down the heavier distillates to
form lighter compounds.
The various fractions obtained after refining are
used for different purposes The gas fraction, like natural gas, is
used chiefly as a fuel for heating. Petrol is used in spark ignition
internal combustion engines that require a fairly volatile fuel.
Kerosene is used as a lighting and cooking fuel in villages, and also
in tractors and jet engines. Diesel is used in diesel engines.
Non conventional Sources of energy
The conventional sources of energy discussed in the previous section are exhaustible and cannot be quickly replaced when exhausted. It takes millions of years for these sources to be formed from the decay of living organisms. These sources are, therefore, also known as non-renewable sources of energy. In contrast, we have another class of the sources such as the sun, wind, waves, tides, and geothermal heat which are inexhaustible. These sources of energy are, therefore, known as renewable sources of energy.
Concept flow
- Unlike fossil fuels and to some extent, forest and biomass, non-conventional sources of energy are renewable and can potentially be used for longer periods of time
- A major problem in harnessing these sources of energy is that the energy released by them is highly diffused as compared with the energy obtained from fossil fuels or nuclear fuels.
- Considerable research is on to make these sources of energy viable.
Solar energy
The source of energy most readily available to us is the sun. Solar energy has several advantages over the other energy sources. It is inexhaustible; it is free from any pollution and unlike fossil fuels, transformation of solar energy does not produce any toxic by-products.
Nature provides some concentration of the sun’s
energy in the form of wind and waves. The gradients set up in the
atmosphere by solar heating turn some of its energy into the movement
of large masses of air, thereby providing wind energy. This wind, in
turn, whips up the waves in the sea which at places can provide
highly concentrated energy. But none of these sources of energy in
their natural form can as yet provide a viable alternative to the
conventional sources. Therefore, global effort is on to tap energy
in concentrated form from the non-conventional sources.
When solar radiation strikes the earth’s
atmosphere, some of it is reflected by dust particles and clouds,
some of it is absorbed by carbon dioxide, water vapour, ozone layer
and the remaining reaches the earth’s surface. Most of the
ultraviolet radiation is absorbed by the ozone layer. Some infrared
radiation is absorbed by the ozone layer. Some infrared radiation is
absorbed by clouds, carbon dioxide and water vapour. The amount of
radiation, reaching the earth, thus may vary with the presence of
clouds, humidity, the latitude- the position of the place north or
south of equator, the time of year, the time of day and other
factors. An idea of the magnitude of energy reaching the earth’s
surface falling on an area equal to the size of the tennis court per
day is roughly equal to the energy obtained from 135 litres of petrol
or 180 kg of coal.
Harnessing Solar Energy
Solar energy can be harnessed in five ways:
- using solar panels
- solar thermal
- concentrated solar power
- solar nanowires and
- By using photosynthetic and biological processes.
However, before solar energy can be successfully utilized, two major problems need to be solved. Firstly solar energy is highly diffused; that is, it is thinly spread over the earth’s surface and so one needs to concentrate it, secondly, solar energy has to be stored for us during night or on a very cloudy day.
When sunlight concentrated by a convex lens is
made to fall on a piece of paper, it burns. The problem of
concentrating solar energy may be solved through the use of different
types of reflectors for focusing sunlight. Reflectors are used in
solar cookers and solar ovens. The simplest of these reflectors is a
single reflector provided in hot-box type of solar cookers. It is a
sheet of polished looking glass or aluminized plastic hinged to one
side of the box which reflects solar radiation into the cooker and
heats it. In a solar oven, several reflectors are provided on all
sides of a box. Curved mirrors, parabolic reflectors and Fresnel
lenses are also used in solar cookers.
Solar Thermal Power Generation
Generally two approaches are followed in this method of power generation
(1)sunlight reflected from several mirrors
arranged in an array is focused on a single heat exchanger in a solar
furnace; and
(2)a large number of cylindrical reflectors in a
solar farm focus solar radiation on long pipes carrying a gas which
collects the heat. A good example of the solar furnace approach is
the tower concept. Sunlight is focused on to a boiler mounted on the
top of a tower located near the centre of the field of mirrors to
produce a high temperature for driving a steam turbine. Another
similar plant system used arrays of heliostat-guided mirrors to focus
sunlight into a cavity-type boiler near the ground to produce steam
for a steam turbine electric power plant. Sunlight striking the
mirrored faces of the heliostat modules is reflected and concentrated
in the cavity of the heat exchanger.
In contrast to the solar furnace approach, in the
solar farm, parabolic cylindrical concentrators or other types of
concentrators are used to focus sunlight on to a central pipe
surrounded by an evacuated quartz envelope. Heat collected by a
fluid(nitrogen or helium) flowing through these pipes may be stored
at a temperature over 500 degree Celsius in a molten salt. This heat
may then be used to drive steam turbines for the generation of
electricity
Photovoltaic Power Generation
 Unlike
the solar thermal systems discussed above, solar cells offer a very
attractive means for direct conversion of sunlight into electricity
with high reliability and low maintenance. The disadvantages,
however, are high cost and difficulty of storing large amounts of
electricity as compared with the relative ease of storing heat for
later use. The working of a solar cell depends upon the phenomenon
of photo electricity; that is, the liberation of electrons by light
falling on a body. Though this phenomenon has been known for a long
time, its application to semiconductors such as silicon has proved to
be of great use. The principle of solar cells is simple. When light
waves strike a semi-conductor material with energy sufficient to
dislodge an electron from a fixed position in the material and make
it move freely in the material, a vacant electron position or ‘hole’
is created in the material. The hole acts a positive charge and can
move if a neighbouring electron leaves its site to fill the hole
site. A current is created if the electron-hole pairs are separated
by voltage in the cell material. Such an intrinsic voltage may be
created by adding small amounts of impurities or dopants to the pure
material or by joining two semiconductor materials. When impurities
such as phosphorous are introduced into silicon, it becomes
electron-rich and is referred to as ‘n-type’ silicon. On the
other hand, impurities such as boron give rise to ‘p-type’
silicon with excess of holes. When these two oppositely charged
semiconductors (one electron rich and the other electron-deficient)
are in contact, free charge leaks across the common boundary and
becomes fixed as ions in the region adjacent to the boundary. At the
interface, the fixed (but opposite) ions create an electric field
that sends free electrons one way and free holes the other.
Unlike
the solar thermal systems discussed above, solar cells offer a very
attractive means for direct conversion of sunlight into electricity
with high reliability and low maintenance. The disadvantages,
however, are high cost and difficulty of storing large amounts of
electricity as compared with the relative ease of storing heat for
later use. The working of a solar cell depends upon the phenomenon
of photo electricity; that is, the liberation of electrons by light
falling on a body. Though this phenomenon has been known for a long
time, its application to semiconductors such as silicon has proved to
be of great use. The principle of solar cells is simple. When light
waves strike a semi-conductor material with energy sufficient to
dislodge an electron from a fixed position in the material and make
it move freely in the material, a vacant electron position or ‘hole’
is created in the material. The hole acts a positive charge and can
move if a neighbouring electron leaves its site to fill the hole
site. A current is created if the electron-hole pairs are separated
by voltage in the cell material. Such an intrinsic voltage may be
created by adding small amounts of impurities or dopants to the pure
material or by joining two semiconductor materials. When impurities
such as phosphorous are introduced into silicon, it becomes
electron-rich and is referred to as ‘n-type’ silicon. On the
other hand, impurities such as boron give rise to ‘p-type’
silicon with excess of holes. When these two oppositely charged
semiconductors (one electron rich and the other electron-deficient)
are in contact, free charge leaks across the common boundary and
becomes fixed as ions in the region adjacent to the boundary. At the
interface, the fixed (but opposite) ions create an electric field
that sends free electrons one way and free holes the other.
In the dark, no current flows in the solar cell.
But when it is illuminated, a current will flow as long as the cell
is illuminated and can supply electricity to an external load.
The
best and most efficient solar cells are constructed from high purity
silicon. This type of cell has already been used very successfully
for providing electrical power in spacecraft. The overall efficiency
of photovoltaic cells is around 11 percent. But their cost has
prevented their use for large-scale generation
Conversion into Electrical Energy
The conversion of solar energy into electricity can be achieved via two routes:
(1) solar energy is
used to boil water which can then be used to generate electricity
(solar thermal power generation)
(2) direct conversion of solar energy into
electricity using solar cells.
Wind Energy
Wind is produced by temperature differences in the air. This is true both of a gentle evening breeze as well as a roaring hurricane. The main driving force behind the wind system of the earth’s atmosphere is the temperature difference between the tropics and the polar regions. This temperature difference arises due to the fact that the tropical regions of the earth are much warmer than the polar regions. Wind energy is the energy of air in motion and has been used for ages for driving sail boats, for grinding grain and pumping water.
Today it is also used
for generating electric power. When air is blown on wind vane, a
child’s common toy, starts rotating. This idea is used in making
the windmill which is nothing but a big wind vane. Wind energy is
inexhaustible and is therefore a permanent source of energy as there
will always be winds. According to an estimate, about 175 to 220
thousand trillion watt hours per year of wind energy can be produced
globally, This is a very promising figure as it is about 2.7 times
the total energy used on the earth today.
Hydroelectric Power
Running water is an easily available source of energy. It is available free and does not pollute the environment. Running water can be used to burn a turbine generator to produce electricity and is the basis of the hydroelectric plant. Water is stored in a reservoir behind a dam. When water flows from a height, it turns big turbines to generate electricity.

Srisailam
Dam across the Krishna River
The gravitational potential emergy of the water is converted into kinetic energy that powers the turbines to produce electricity. There are a number of hydroelectric power plants in India; the Bhakra Nangal dam in punkab, Damodar valley Project in West Bengal, Hirakud Project and Kosi Project in Bihar and the Nagarjunasagar project in Andhra Pradesh to name a few. These projects produce a very significant percentage of the total electricity generated in our country. However, large dams also have a high degree of environmental impact in terms of flooding and silting of the rivers. The environmental costs of the dams must be weighed against the benefits of the dams.
Ocean energy
Tides are the raising and filling of the ocean level due to the moon’s gravitational pull which can be easily seen along the seashore. It is possible to extract energy from these tides and the main requirement for harnessing tidal energy is that the difference between the high tide and the low tide should be large, at least a few metres. The first ever tidal powered electric generating plant was set up on River Rance in France, to harness the power tides in the English Channel which rise to as much as 14 metres at this location. By opening gates as the tide rises and then closing them at high tide, a 23-square kilometers pool is formed behind the Rance river dam. As the tide falls, the trapped water is allowed to flow out, driving 24 electricity generating turbines of 13MW capacity each for total average power output of 310MW.
It is estimated that
the total global potential for tidal power is only2 percent of the
world’s potential hydroelectric capacity. Besides, it involves
very high initial cost and the output is variable.
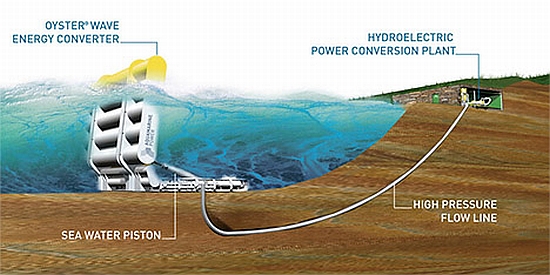
Energy from waves
Unlike the tides which exhibit a regular but long periodic variability, waves keep the ocean water in continual motion. The vertical rise and fall of the successive waves can be used to produce energy. India’s first wave energy project has gone on stream at vizhinjam near Trivandrum. The 150-MW project implemented by the Wave Energy Group attached to the Ocean Engineering Centre, IIT, Madras, in association with the state Harbour Engineering department. This project works works on the principle of an oscillating water column, which drives an air turbine. The turbine is so designed that it rotates in one direction, irrespective of the direction of air flow. It is a multipurpose scheme and floats on the sea bed. From the ecological and environment points of view too, it is the best as the unit hardly leaves any waste. The biggest advantage of the project is that power generation is possible throughout the year, though not uniformly.
|
|
|
Energy from Ocean Thermal Gradients

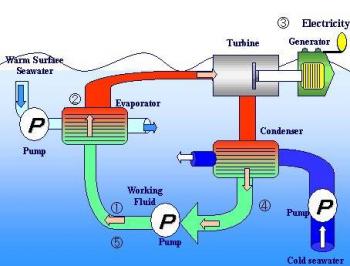 The
heat contained in the ocean waters heated by the sun can be converted
into electricity by utilizing the difference in temperature between
the surface water can be used to heat some low-boiling organic liquid
such as ammonia or propane, the vapours of which are allowed to
expand through a turbine which can run a generator. The vapours
leaving a turbine are channeled into a condenser located in the low
temperature water zone, much below the water surface. The condensed
liquid is pumped into a boiler evaporator to start the cycle afresh.
The
heat contained in the ocean waters heated by the sun can be converted
into electricity by utilizing the difference in temperature between
the surface water can be used to heat some low-boiling organic liquid
such as ammonia or propane, the vapours of which are allowed to
expand through a turbine which can run a generator. The vapours
leaving a turbine are channeled into a condenser located in the low
temperature water zone, much below the water surface. The condensed
liquid is pumped into a boiler evaporator to start the cycle afresh.
The expected efficiency
of such a system is about 2 percent. The amount of energy available
from ocean thermal gradients is enormous, and is replenished
continuously.
Geo thermal energy
We are living between two great sources of energy- the sun up in the sky, and hot rocks beneath the earth’s surface. The interior of the earth is extremely hot. As one goes deep into the earth the temperature increases. The earth has very hot materials in and below the crust. As some places this hot material comes quite close to the surface, or even out of the surface in the form of volcanic eruptions. In places where it comes close to the surface, underground water often gets heated and produces steam and hot water which comes out as hot springs and geysers. The areas in which such conditions exist are known as geothermal regions. Infrared photography of the ground from satellites like IRS-1 has played an important role in locating the geothermal regions.
 After
locating a geothermal region, a bore is drilled through the rock to
tap the heat. In many places steam under high pressure comes out
straight from the borehole and can be used to drive turbines
directly. Where steam is not available, cold water is pumped down
through one hole and heated water and steam produced pumped out of
another. The hot water or steam produced can be used to run a turbine
or to heat housed and offices. Geo thermal energy is used for space
heating where the temperature goes down to 30 to 35 degrees below the
freezing point, for poultry farming, mushroom cultivation and
pashmina-wool processing which need a warmer climate.
After
locating a geothermal region, a bore is drilled through the rock to
tap the heat. In many places steam under high pressure comes out
straight from the borehole and can be used to drive turbines
directly. Where steam is not available, cold water is pumped down
through one hole and heated water and steam produced pumped out of
another. The hot water or steam produced can be used to run a turbine
or to heat housed and offices. Geo thermal energy is used for space
heating where the temperature goes down to 30 to 35 degrees below the
freezing point, for poultry farming, mushroom cultivation and
pashmina-wool processing which need a warmer climate.
Although at first
glance, geothermal energy seems very promising, geothermal sources
are, in reality, far from being pollution free. Underground steam
contains hydrogen sulphide gas and minerals which can poison fish and
other forms of aquatic life in streams and rivers.
Energy storage
We have seen that whatever the source of energy, all of these are converted into electrical energy for use. This brings to focus the need for energy storage and distribution.
One of the problems associated with the generation
of electricity from various is that the demand for power fluctuates.
During the day, the power requirements, especially for commercial
purposes, are much greater than during night time. If there was some
way to store electrical energy, the generating plant could be
operated at full capacity during the night, storing up energy to be
released when the demand increases.
Concept flow
Some of the key ideas in energy storage are:
- All forms of energy are used to generate electrical energy
- Generation, storage and transmission of electrical energy is involved in meeting energy demands
- There are two different approaches for storing electricity energy, depending upon whether this storage is for small-scale or large-scale purposes.
Small-scale storage
For
small-scale storage of electricity, batteries and fuel cells offer
the best course. An electric battery consists of one or more electric
cells which are devices for producing electric directly from the
chemical reactions. Electricity made in this way is much more
expensive than that made by mechanical generators but batteries being
compact and portable, are a better choice for many applications
Cells are generally of two types; primary cells and secondary cells.
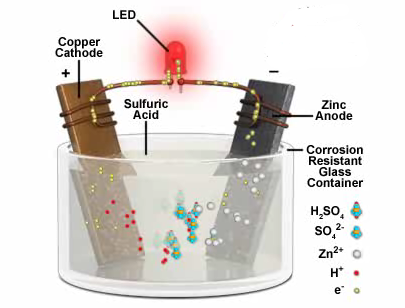
One good example of a primary cell is a voltaic
cell which consists of copper
and zinc metal plated dipped in a solution of
sulphuric acid which acts as an electrolyte. The electrolyte
contains positive and negative ions but is overall electrically
neutral. Zinc tends to dissolve in the solution more easily than
does copper. In solution it forms positive zinc ions leaving
electrons behind on the zinc plate. This gives zinc plate a negative
charge with respect to copper plate and so, when an electric circuit
is completed at the terminals of the cell, the electrons flow to the
copper plate giving rise to a current. This cell becomes dead when
the solution becomes saturated with zinc so that no more of it wants
to dissolve. To revive the cell one has just to replace the
electrolyte sulphuric acid, this can be continued so long as zinc is
present but when the zinc is gone, the cell is permanently dead. The
principal disadvantage of the primary cell is its short life.
Obviously it would be more useful to have a cell which could be
restored to its original condition once it had discharged all its
available form of energy. Fortunately it is possible to do so with
some types of cells. Such cells are called secondary cells and a set
of them is called a storage battery because it enables charges
produced by other means to be stored for later use. In a storage
battery electricity is stored where chemical changes take place which
can be reversed to release the stored electrical energy. A typical
storage battery takes a few hours to charge and can then be used as a
source of energy.
These batteries find use in the fabrication of
uninterrupted power supplies for continuous operation of costly
equipment, computers and other strategic gadgets. Here the batteries
are coupled with an oscillator which converts this D.C supply from
this battery to A. C. at higher voltage and frequency.
Large scale storage
For meeting the large energy requirements of homes and industry, storage batteries are impractical. To meet the requirements of varying demands, electricity generation is increased or decreased and in many cases, there is a transmission and distribution company that manages this kind of scheduling.
Nuclear energy
We have seen how different sources of energy are used to generate steam and hence the mechanical energy to produce electricity. These sources can be renewable or non-renewable. Yet another source that has been used to generate electrcity is nuclear energy, the atomic energy is used in the generation of energy. In this section, we will understand the atomic structure and the source of energy in the atom.
Concept flow
- Atoms consist of protons, neutrons and electrons. Different atoms and nuclei have different levels of stability. Atoms occur in more than one configuration due to difference in their nuclear structure and these different configurations are called isotopes.
- A vast amount of energy is available within the nucleu and nuclear reaction processes release this energy. Nuclear energy so produced is used in the place of coal or gas to heat water and produce steam. A nuclear reaction occurs when uranium atoms split intosmaller particles in a chain reaction that produces a large amount of heat.
- Chemical reactions, such as burning, involve the release of energy mainly due to exchange or transfer of electrons whereas nuclear energy involves the release of energy from within the nucleus of the atoms.
Atoms
All matter is made up of different naturally occurring elements, each possessing physical and chemical properties distinct from the other. These smallest entities into which these elements can be divided are called atoms. Atoms are made up of three still smaller particles-positively charged protons, negatively charged electrons and electrically neutral neutrons. The relatively heavy protons and neutrons constitute the tiny nucleus. This is surrounded by moving electrons and overall the orbit is electrically neutral and is about ten to the power of 5 times the size if the nucleus. The number of orbiting electrons is always equal to the number of protons so that the net electrical charge of the atom is Zero.
Atoms of a particular element always contain the
same number of protons and there is a scale of elements, going from
the atoms of hydrogen which have only one proton, helium atoms which
have two protons, lithium atoms which have three protons, and so on
all way up to very large atoms such as uranium, which has 92 protons.
The number of protons is therefore, important because it identifies
the element to which the atom belongs. Thus any atom which contains,
say 17 protons must be chlorine; uranium atoms always contain 92
protons, and so on. The atomic number of an element is the same as
the number of protons in the nucleus.
 Along
with the protons in the atomic nucleus there are also the
electrically neutral particles called neutrons. The number of
neutrons in the nucleus tends to increase with number of protons.
For instance, in the helium atom ( which has two protons) there are
two neutrons, lithium (which has three protons) has four neutrons,
uranium (which has 92 protons) has 146 neutrons, and so on. The
protons and neutrons in a nucleus are bound together by immensely
strong forces, called nuclear forces which counteract the
electrostatic forces of repulsion acting between the protons. In
very large atoms, such as uranium and plutonium, the binding nuclear
forces are only slightly stronger than the repulsive forces between
the large number of protons; such nuclei are unstable and can be made
to undergo fission- split into two or more fragments releasing
nuclear energy.
Along
with the protons in the atomic nucleus there are also the
electrically neutral particles called neutrons. The number of
neutrons in the nucleus tends to increase with number of protons.
For instance, in the helium atom ( which has two protons) there are
two neutrons, lithium (which has three protons) has four neutrons,
uranium (which has 92 protons) has 146 neutrons, and so on. The
protons and neutrons in a nucleus are bound together by immensely
strong forces, called nuclear forces which counteract the
electrostatic forces of repulsion acting between the protons. In
very large atoms, such as uranium and plutonium, the binding nuclear
forces are only slightly stronger than the repulsive forces between
the large number of protons; such nuclei are unstable and can be made
to undergo fission- split into two or more fragments releasing
nuclear energy.
Isotopes
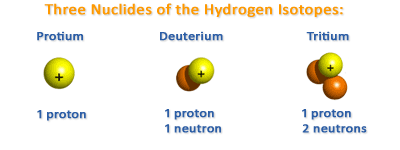
The atomic weight of an element is expressed as the sum of the number of protons and neutrons. Atoms of the same element can differ from one another by having a different number of neutrons in their nuclei. For example, uranium (which has 92 protons) can have either 143 or 146 neutrons. These two forms of uranium have the same chemical but slightly different physical properties. Atoms of the same element which exist in different forms as a result of having different numbers of neutrons in their nuclei are called isotopes. The first of the two uranium isotopes described above is called uranium-235, and the second, uranium-238. Uranium-238 is heavier than uranium-235. Similarly there are three isotopes of hydrogen, namely hydrogen (one proton only), deuterium or heavy hydrogen ( one proton and one neutron), and tritium ( one proton and two neutrons).
Atoms of the same element always contain the same
number of protons in their nuclei. The number of protons is balanced
by an equal number of electrons to make the atom electrically
neutral. It is the number of orbiting electrons that determines the
chemical properties of an element. When atoms combine with other to
form molecules, there is rearrangement of the outermost electrons in
the orbit ( also called valence electrons). In this process, energy
can be stored or released so that is some reactions ( burning for
example) heat energy is released. Thus atoms of carbon ( coal, on
combination with atoms of oxygen from the air to form carbon dioxide
in the process of burning, release energy.
Principle of Nuclear Fission
 Isotopes
of certain very heavy atoms, for example, uranium-235 and
plutonium-239, are so unstable that they can be made to split
(fission) when hit by a neutron. When this happens, a very large
amount of energy is released. This process is known as nuclear
fission. In this process, the mass of the two smaller atoms (
collectively known as fission products) plus the mass of the two or
three neutronsa that are produced, is slightly less than the mass of
the original uranium-235 or plutonium-239 atom plus the bombarding
neutron. Thus, in the process of nuclear fission, some matter is
lost and converted into energy. According to Einstein’s famous
equation, even a small amount of matter is equivalent to a very large
amount of energy. Thus the nuclear fission of uranium-235 contained
in 1 tonne of natural uranium is equivalent in electricity output to
the burning of approximately 20,000 tonnes of coal. Natural uranium
contains only 0.7 percent of uranium-235.
Isotopes
of certain very heavy atoms, for example, uranium-235 and
plutonium-239, are so unstable that they can be made to split
(fission) when hit by a neutron. When this happens, a very large
amount of energy is released. This process is known as nuclear
fission. In this process, the mass of the two smaller atoms (
collectively known as fission products) plus the mass of the two or
three neutronsa that are produced, is slightly less than the mass of
the original uranium-235 or plutonium-239 atom plus the bombarding
neutron. Thus, in the process of nuclear fission, some matter is
lost and converted into energy. According to Einstein’s famous
equation, even a small amount of matter is equivalent to a very large
amount of energy. Thus the nuclear fission of uranium-235 contained
in 1 tonne of natural uranium is equivalent in electricity output to
the burning of approximately 20,000 tonnes of coal. Natural uranium
contains only 0.7 percent of uranium-235.
Fission Nuclear Reactors
A nuclear reactor is a device in which the nuclear fission process is carried out under controlled conditions. The basic fuel of a nuclear reactor is uranium, obtained from ores such as pitchblende. Natural uranium is a mixture of two isotopes, uranium-235 and uranium 238, present in the ratio of 0.7:99. Only the comparatively rare isotope uranium-235 undergoes nuclear fission.
There are two types of nuclear reactors:
- Thermal reactors use uranium as fuel and have moderators to slow down neutrons. They are called thermal reactors because they use slow-moving or thermal neutrons.
- Fast breed reactors: Since 99.3 percent of natural uranium is uranium-238 and since this isotope cannot undergo fission, thermal reactors are able to use a very small proportion of natural uranium. However, fast reactors are able to convert this otherwise unusable uranium-238 into a new element, plutonium-239, which can then be fissioned to liberate energy. Fast breeder reactors do not need a moderator as fast neutrons used.
Thermal reactors
The core of a thermal has five components. Uranium dioxide pellets are packed in long metal tubes known as fuel elements. A nuclear reactor contains several tones of uranium in thousands of fuel elements. Periodically, the used-up fuel elements are taken out and new fuel elements put in. The most commonly used moderators used moderators are water, heavy water, and graphite.
Control rods are used to control the rate of the
nuclear chain reaction. They are made of boron which readily absorbs
neutrons. When these rods are lowered into the core (containing fuel
elements and the moderator), that absorb most of the neutrons and so
there can be no chain reaction. This effectively shuts down the
reactor. As they are pulled out progressively, neutrons are
available to split uranium atoms, thus releasing more neutrons. The
further the control rods are pulled out, the larger is the number of
fissions in the core and more heat is produced.
Since the uranium fuel as well as the fission
products are intensely radioactive, a very thick steel-and-concrete
shield is required to prevent the escape of any radiation from the
core. Indian reactors usually have double shielding to further
minimize any risk.
To remove the heat produced by nuclear fission in
the fuel elements, a coolant is used which circulates through spaces
between the fuel elements, Indian reactors, which use natural
uranium as fuel, use heavy water as coolant, but reactors using
enriched uranium (in which the percentage of uranium-235 is raised by
complex processes) use ordinary water as coolant. The heated coolant
coming out of the core transfers the heat exchanger to boil water and
raise steam which is then used to run a turbine generator to generate
electricity.
Fast breed reactors
Thermal reactors are able to release energy from the small proportion of uranium-235 contained in the natural uranium. They are, however, unable to use the uranium-238 which constitutes 99.3 percent of natural uranium. The significance of fast reactors is that they are able to convert Uranium-238 into plutonium-239 in significant quantities, so that much into plutonium-239 in significant quantities, so that much more energy can be extracted from natural uranium than is possible with thermal reactors.
There are two important features of fast breeder
reactors. Firstly, there is no moderator. The neutrons given off in
the fission reaction are not slowed down. ( It is for this reason
that this type of reactor is known as a fast reactor.
Secondly, the fuel elements of fast reactors
contain a mixture of plutonium-239 and uranium-238. Plutonium is
placed in the centre of the core, whereas the uranium-238 is located
in a blanket surrounding the plutonium core. Two processes take
place simultaneously in these reactors:
(i)Plutonium-239 (originally produced from some of
the uranium-238 atoms) in thermal reactors is fissioned, producing
heat which is removed by the coolant. Since the heat produced in the
core is very large, the coolant used in a fast reactor is liquid
sodium.
(ii)A significant proportion of uranium-238 is
converted into plutonium in the blanket. In fact more plutonium is
bred in the blanket than is fissioned in the core, and for this
reason, fast reactors are known as fast breeder reactors.
Plutonium-239 atoms are created when uranium-238 atoms absorb fast
moving neutrons.
Spent fuel
In both thermal and fast reactors, the spent fuel elements contain three types of material: (i)highly radioactive fission products; (ii)large amounts of unused uranium-238, known as ‘depleted’ uranium; and (iii) a certain among of plutonium. By reprocessing the fission products from spent fuel, the plutonium and depleted uranium can be fabricated into new fuel elements for fast reactors. By repeated processes through fast reactors followed by reprocessing, it is possible to extract much more energy than when using only thermal reactors. 1 tonne of natural uranium fissioned in a thermal reactor is equivalent to about 20,000 tonnes of coal. Used in fast reactors, however, 1 tonne of natural uranium is equivalent to about 1,000,000 tonnes of coal.
Nuclear Fusion
What is happening inside the sun?
 The
sun consists mainly of hydrogen gas. The atoms of hydrogen under
tremendous pressure at the centre of the sun, come together and fuse
to for helium nucleus along with the liberation of tremendous energy
in the form of heat and light. It this energy which maintains its
temperature and makes the sun shine. In this process again, like in
nuclear fission, mass is converted into energy. This is also the
principle on which the hydrogen bomb is based. Since the various
reactions taking place inside the sun occur at very high
temperatures, they are called thermonuclear reactions. The sun,
therefore, may be considered as a thermonuclear furnace where
hydrogen atoms are continuously being fused into helium atoms. Mass
lost during these fusion reactions Is converted into energy.
The
sun consists mainly of hydrogen gas. The atoms of hydrogen under
tremendous pressure at the centre of the sun, come together and fuse
to for helium nucleus along with the liberation of tremendous energy
in the form of heat and light. It this energy which maintains its
temperature and makes the sun shine. In this process again, like in
nuclear fission, mass is converted into energy. This is also the
principle on which the hydrogen bomb is based. Since the various
reactions taking place inside the sun occur at very high
temperatures, they are called thermonuclear reactions. The sun,
therefore, may be considered as a thermonuclear furnace where
hydrogen atoms are continuously being fused into helium atoms. Mass
lost during these fusion reactions Is converted into energy.
There are several possible reactions in which
light atoms can fuse to form heavier nuclei and release energy, but
the one which the scientists which have been trying to accomplish is
the thermonuclear reaction involving deuterium and tritium nuclei and
not hydrogen nuclei. This is due to the fact that though ordinary
hydrogen is the raw material for the thermonuclear process in the
sun, its reaction rate is quite slow. Reactions involving deuterium
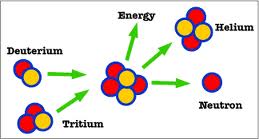
nuclei or deuterium and tritium nuclei are more efficient. Fusion of
two nuclei of deuterium of deuterium forms a tritium and a hydrogen
nuclei while the fusion of a deuterium and a tritium nuclei forms a
helium nucleus with two protons and two neutrons.
For the above reactions to take place, the
colliding deuterium nuclei should have enormous speed. This is made
possible by heating the particles to a few hundred million degrees.
Remember, much below this temperature, the atoms are already stripped
of their electrons. Thus they form a mixture of positively charged
ions and electrons known as plasma.
If one can fuse all the nuclei in 1 gram of
deuterium, it would yield 100,000 kWh of energy. A complete fission
of an equivalent amount of uranium, on the other hand will give
25,000kWh.
Fusion Reactors
Despite its tremendous potential there are many technical problems in building a practical fusion reactor. One major problem is the confinement and control of plasma at more than a hundred million degrees so that thermonuclear energy could be made available at a steady rate. One very successful method to confine the plasma in a magnetic field.
Among other alternatives being tried for
harnessing nuclear fusion is one by using lasers. A laser is a
highly powerful beam of coherent beam of coherent light which can be
focused on a very small spot. In this method, called inertial
fusion, pellets of deuterium-tritium fuel are rapidly compressed and
heated by bombardment with laser beams, resulting in a series of
miniature thermonuclear explosions and production of energy.
One of the most serious problems in the nuclear
fusion process is the fact that large amounts of tritium is only
weakly radioactive, its chemical behavior is exactly the same as
ordinary hydrogen and it can readily enter into organic substances.
Control of tritium will be one of the major problems in the operation
of the fusion reactors.
There are many advantages of fusion power. The
fuel supply is plentiful and relatively inexpensive.
The world’s oceans constitute an inexhaustible
source of the primary fuel deuterium in the form of water; about one
molecule out of every 3,000 water molecules contains an atom of
deuterium. The products of fusion reactions are either stable
isotopes or they are only weakly radioactive. Radioactivity will
also be produced by the neutrons released in the reactions when they
are captured in the materials of the reactor.
Further, fusion reactors do not produce air
pollutants that contribute to acid rain or global warming. Despite
these advantages, however, immense difficulties are yet to be
overcome before energy generation can become feasible on a large
scale.
The process of nuclear fission involves the
splitting of a heavy nucleus while the nuclear fusion is the joining
together of lighter atoms to form heavier ones. Both the processes,
however, release tremendous amounts of energy.
Energy and the environment
Modern society cannot exist without the production and utilization of energy. Every month we have to pay direct charges for use of electricity. Oil and gas in our homes and for the petrol used in our cars. And there are also indirect charges that we pay for the energy used in manufacturing processes and for the transportation of the goods that we buy. In addition to these charges, we pay also in terms of the effects that energy production and energy utilization have on our world in terms of environment pollution. Environmental pollution may be defined as the unfavorable alteration of our surroundings. It may not be possible to estimate monetary losses or many of the side effects associated with energy production and energy utilization. What is the value of the health impairment, for example, caused by the cars exhaust fumes? What value do we place on the destruction of farmland and pollution of water caused by strip mining for coal? What value is associated with the loss of seaside beaches because of oil pills washing ashore? As a matter of fact, as long as we continue to produce and utilize energy, we will have to pay for these undesirable side effects. How much are we willing to pay?
Concept flow
- Uncontrolled energy consumption places a strain on the environment
- Mining for coal and drilling for petroleum leads to destruction of land, pollution and habitat loss
- What are the ecological costs of oil spills, health lost and other such effects?
- There needs to be a judicious use of energy
Threats from Fossil fuels
Most of the energy that is generated throughout the world at present is derived from the burning of fossil fuels-coal, natural gas and petroleum products. There are numerous environment problems associated with the extraction, transportation and utilization of fossil fuels.
The most plentiful fuel source in the world is
coal. The highest quality coal(anthracite generally occurs
sufficiently far underground to require high-cost deep-mining
techniques. Further, anthracite generally contains a very high
percentage of sulphur and it cannot be used as a fuel without
expensive treatment to remove sulphur. Consequently in recent years,
there has been increased interest in the mining of lower quality but
relatively sulphur-free coal that lies close to the surface.
Strip-mining techniques are used for the extraction of this coal.
Strip mining for coal causes serious and continuing environmental
problems. One of the most serious problems associated with the
strip-mining of coal is the huge amount of land that is torn up in
the process. Unless rehabilitation measures are taken, the area
adjoining the strip mined land can suffer from landslides, erosion
and sedimentation.
Unlike coal, the extraction of oil does not
desecrate the land the way the strip-mining does. However, the most
serious environmental problem associated with oil-well drilling
occurs at offshore sites. Because of the many technical difficulties
inherent in offshore drilling, if a rupture occurs or if the drilling
opens a crack in the rock that contains the oil deposit, a major
leakage of oil into the water can occur before the damage is repaired
or the crack is sealed. The release of large amounts of oil into the
water can be injurious to the marine life. When the oil spreads over
water, the diffusion of oxygen into water is inhibited. This affects
the respiration of fish and other marine life. Oil pollution of sea
causes either problems too. Oil is pushed to the shore by the water
currents and winds, thereby spoiling the beaches.
Combustion of fuel
The burning of fossil fuels releases a variety of noxious gases and particulate matter into the atmosphere. The major contributors to this atmospheric pollution are coal and oil and natural gas by far is the least offensive of the fossil fuels , One of the major problems with coal and oil is the presence of sulphur. Depending upon the source, the sulphur content can be up to several percent and upon combustion several oxides (particularly sulphur dioxide) are produced. When sulphur dioxide is released into the atmosphere, it combines with water vapour and forms sulphuric acid. It is this sulphuric acid which is injurious to plant and animal life. It has been found that atmospheric sulphuric acid eating the limestone facings of many monuments and public buildings in urban life. Sulphur dioxide is believed to cause cough, shortness of breath and spasm of the larynx. It can cause acute irritation to the membranes of the eyes resulting in excessive flow tears and redness. When absorbed by plants beyond a certain level the plants cells become inactive and are killed, resulting in tissue collapse and drying of leaves. Sulphur dioxide is also known to interfere with the respiratory and photosynthesis in plants.
 The
burning of petrol in internal combustion engines is the major source
of carbon monoxide, nitrogen dioxides and hydrocarbons in the
atmosphere. In addition, there are large quantities of lead which
are released into the atmosphere from high octane petrol used in
cars. All these pollutants and the products of the photochemical
reactions they undergo in presence of sunlight contribute to the
noxious known as smog. There seems at present no escape from the
health hazards of smog until some effective way is found to remove
the pollutants from the vehicular exhaust gases.
The
burning of petrol in internal combustion engines is the major source
of carbon monoxide, nitrogen dioxides and hydrocarbons in the
atmosphere. In addition, there are large quantities of lead which
are released into the atmosphere from high octane petrol used in
cars. All these pollutants and the products of the photochemical
reactions they undergo in presence of sunlight contribute to the
noxious known as smog. There seems at present no escape from the
health hazards of smog until some effective way is found to remove
the pollutants from the vehicular exhaust gases.
Combustion of fuel - Effects of carbon Dioxide and carbon Monoxide:
The consumption of oxygen and the formation of carbon dioxide are necessary consequences of every combustion process. One may think that this may deplete the world’s supply of oxygen and thus upset the oxygen-carbon dioxide balance that is necessary for plant and animal life.
Carbon dioxide molecules strongly absorb heat
radiations emitted from the surface of the earth heated by the sun.
By holding back this energy in the earth’s atmosphere, carbon
dioxide reduces the heat lost by the earth to space. This is called
‘greenhouse effect’ and because of this, it is argued, the
continued burning of fossil fuels will result in a steady increase in
the earth’s surface temperature. However, an increasing in the
temperature of the earth’s surface and lower atmosphere has the
compensating effect of increasing evaporation and cloudiness.
Because clouds reflect some of the incident sunlight, increases in
cloudiness tend to decrease the surface temperature. Further, the
release of particulate matter into the atmosphere from fuel burning
increases the number of condensation sites around which water
droplets can form. The result is an increase in the amount of rain,
hail and thunderstorms which lead to the lowering of the temperature.
The amount of carbon dioxide is regulated by the presence of the
ocean waters which 60 times as much carbon dioxide as does the
atmosphere and absorbs a large fraction of the carbon dioxide
released by the burning of fuels. Also, the increased level of
carbon dioxide in the atmosphere actually stimulates a more rapid
growth of plants. This increased utilization of carbon dioxide
further reduces the atmospheric excess. Thus the role of carbon
dioxide in influencing the world’s climate is quite a complex one.
Carbon monoxide is another pollutant produced by
burning of fossil fuel. It is usually produced when there is
insufficient oxygen for burning. It is released into the atmosphere
mainly from automobile exhaust gases. But it does not so far
constitute a serious environmental problem.
Thermal pollution
The term ‘thermal pollution' basically refers to the detrimental effects of discharges of unwanted heat into the environment. All electricity generating plants produce electricity by driving huge turbine generators with steam. The steam is condensed in a cooling system and is cycled back to the heating unit for reuse. The cooling system can be water that is pumped from some nearby reservoir and discharged back into it, or it can be a cooling tower in which the heat is dissipated into the atmosphere. Both cause thermal pollution. If the heated water is discharged into a static reservoir, such as a lake, the effect can be even more severe. The thermal is generated by the energy producer as well as the energy user. Almost all of the energy we use is eventually converted into heat. Most of this waste is dissipated into the air where it contributes to the general atmospheric heating.
Effects of Nuclear Radiations
Nuclear reactors, unlike the other sources of power, offer a lot of advantage. Nuclear reactors generate electrical power without the smoke and fumes that are characteristic of fossil fuel-burning plants. Also the mining of uranium produces much less degradation of the countryside than the mining of fossil fuels, particularly coal. Nuclear reactors, therefore, offer the prospect of long term relatively clean power. However, nuclear reactors have their own peculiar set of disadvantages, mainly associated with the production of radioactive materials. Some radioactive waste is released into the environment both gases into the atmosphere and in the form of low activity waste such as tritium in cooling water.
All radioactive substances emit harmful
radiations, some of which can cause cancer in man and animals and
damage the genetic material of the cell, producing long term harmful
effects in living organisms. However, modern nuclear reactors are
quite safe. An individual living near a nuclear reactor is exposed
much less to its emitted radiation than what one gets from X-rays and
natural sources.
Energy and the future
The worldwide demand for energy is increasing day by day. The increasing use of modern means of transport-cars, buses, trains, aero planes , ships, etc., the rapid rise in the overall industrialization; the tremendous growth in population, particularly in the last 40 years, are some of the factors that have led to a tremendous spurt in mankind’s energy requirements.
Need for Judicious Use of energy
It follows therefore that mankind has to adopt a judicious approach towards consumption of energy sources to ensure that these are not depleted too fast. This approach needs to be supplemented by optimum utilization of our natural sources. We have, for example, reserves of billions of tones of coal spread across the Bihar, West Bengal and Orissa region. This coal may not be of the best quality, but coal mining in this area can always be stepped up to meet our energy requirements. In India, technology used is coal mining and handling after it is mined is still primitive where mechanical wheels are used in open pit mining. Any improvement in material handling system can lead to a saving of a lot of coal which is otherwise lost.
One source of energy which has remained
underutilized is the hydroelectric energy. The subcontinent has many
large rivers with substantial hydroelectric potential, much of which
still remains unutilized. These can be tapped to provide energy
which is clean, renewable and cheap. Large numbers of small
hydroelectric power projects across the country over the country over
small rivers could also yields a fair amount of energy.
Wind energy has a tremendous scope as alternative
source of energy not only in India but the entire region stretching
from Afghanistan to Vietnam. Wind electric generators are at present
operating successfully in many parts of India. Windmills are also
being used for pumping water and this use of windmills should be
encouraged. If India develops a system whereby windmills and
generators could be manufactured on a large scale, it will really be
a tremendous boon to the rural economy of this vast region. Wind
energy is a non-polluting, cheap, renewable source of energy.
A substantial portion of our energy requirements
is met by firewood. It necessitates felling of trees, resulting in
deforestation, soil erosion, and floods. To prevent this and to
maintain the stability of forest reserves a massive afforestation
programme is necessary. The use of firewood as fuel must be avoided
as far as possible by encouraging the use of biogas plants. Benefits
accruing from biogas plants are immense and manifold. Biogas plant
generate but only substantial economic gains to the country but also
help up gradation of the environment. As India is dependent on
imported oil for meeting its energy requirements, it would be prudent
to reduce the consumption of petroleum products. These are primarily
used for road and rail transport. The industry uses a large quantity
of petroleum products both as raw material and also as fuel. There is
tremendous scope for reducing the consumption of diesel and petrol in
cars, trucks and two wheelers by more efficient engine design and
maintenance.
It is indeed a good news that India has vast
reserves of natural gas which is a very clean source of energy. The
Bombay High oilfields contain very large quantities of gas which at
present are flared or burnt. Only recently are efforts being made to
utilize natural gas commercially, for generating power and production
of fertilizer. Especially in the north east. The Dutch and the
British have found vast reserves of offshore natural gas and in the
process have developed new technology to utilize it.
Minimizing Wastage
Not only have we to adopt a judicious approach to using our energy sources, we have also to lay a great stress on prevention of wastage. Even a casual look at our day-today activities reveals that energy is wasted in many ways. Careless habits, like leaving the lights and fans on when no one is round, keeping the car or scooter engine on while gossiping with a friend on the road, etc. contribute to wastage of energy. We have to know about the various ways in which energy is wasted at home and in industries, and then develop-and encourage others to develop-proper design and also ensure that all machinery is kept well maintained and in proper running condition. This helps save a lot of energy. With the impending energy crisis facing mankind, saving ‘every bit of energy ‘ is of great importance. This saved energy can then be put to some useful ‘use’ in future. WE must remember energy saved is energy produced.
Evaluation:
- What is work?When do we say that work is done?
- What are kinetic and potential energy?
- What are the different forms of energy?
- What is power?
- What are the units of energy?
- What are fossil fuels?How are they formed?
- What are the different steps to process the petroleum?
- What is Biomass energy? How it is generated?
- What are the different sources of non -conventional energy?
- What are the different ways of harnessing solar energy?
- Name the different non conventional sources of energy.
- Name the different storage devices.
- What are isotopes? Name some isotopes.
- Draw the diagrams of the nuclear reactors-thermal and fast breed
- How are fossil fuels threatening us?
- What are the effects of Carbon Dioxide and Carbon Monoxide?
- How are nuclear radiations affect out environment?
- What is the need for judicious use of available energy?
- List some steps to minimize energy wastage
Additional web resources
- [[5]]
- File:Energy for KOER html m145a4ee8.gifFile:Energy for KOER html mb374f76.gif[[6]]
- [[7]]
- web.mit.edu/8.02t/www/materials/modules/ReviewD.pdf – This review document summarizes the law of conservation of energy very clearly
- [[8]]
- [[9]]
- [[10]]
- www.sciencejoywagon.com/physicszone/05work-energy/
- www.technologystudent.com/energy1/less4.html
- [[11]]
- [[12]]
- [[13]]
- [[14]]
- www.indiacore.com/.../kssidhu-non-conventional-energy-resources.pdf
- [[15]]
- [[16]]
- uw.physics.wisc.edu/~himpsel/wires.html
- hyperphysics.phy-astr.gsu.edu/hbase/nucene/fission.html
- phet.colorado.edu/en/simulation/nuclear-fission
- www.whatisnuclear.com/articles/nucreactor.html
- [[17]]
- [[18]]
- [[19]]
- www.whatisnuclear.com/articles/nucreactor.html
- www.westinghousenuclear.com/.../WhatIsNuclearEnergy.shtm
- www.indianuclearenergy.net
- video.nationalgeographic.com/video/.../energy.../alternative-energy.html
- en.wikipedia.org/wiki/''Waste''_minimisation - Cached – Similar
- www.youtube.com/watch?v=FBTXQV7GKow
- www.streetdirectory.com/.../'energy'-'crisis-in-india'-aouac.html - Cached – Similar
 Keywords
– Work, Energy, Power, Kinetic Energy, Potential Energy, Law of
Conservation of Energy, Thermodynamics, Biomass, Fossil Fuel,
Combined Cycle Power Generation
Keywords
– Work, Energy, Power, Kinetic Energy, Potential Energy, Law of
Conservation of Energy, Thermodynamics, Biomass, Fossil Fuel,
Combined Cycle Power Generation