Ionic compounds conduct electricity in liquid state
Activity No # 1 - Ionic compounds conduct electricity in liquid state
Shared By :
Sowmyalatha.K
G.H.S.Mudushedde
Mangalore South,D.K.
Estimated Time
10 minutes
Materials/ Resources needed
- 3 pieces of insulated copper wires, each 4-6 inches (10-15 cm) long
- plastic cup
- 9-volt battery
- Bulb
- eye protection (goggles or safety glasses)
Prerequisites/Instructions, if any
Observe carefully. If the light bulb does not light up, make sure all wire connections are tight.
Multimedia resources
Website interactives/ links/ simulations
https://www.youtube.com/watch?v=LkM-a6SwbkE
Process (How to do the activity)
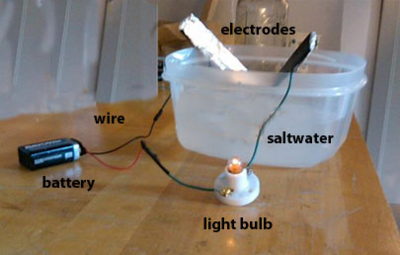
Saltwater Circuit — A saltwater circuit consists of a battery, wire, light bulb, light bulb socket, and two electrodes . When the battery is connected and the electrodes are touched together we have a closed circuit and electrons flow from the positive terminal of the battery to the negative terminal of the battery. This flow causes the light bulb to light up. When the electrodes are not touching, the circuit is "open" and electrons do not flow; this is called an open circuit. In our saltwater circuit, the electrodes act as a switch.
If you submerge the electrodes in common salt, the light bulb does not turn on because no medium exists to transfer electrons . But if you submerge the electrodes in saltwater, the light bulb turns on. In addition, the amount of salt in the saltwater solution influences how much current flows through the circuit, and in turn, how bright the light bulb glows.
Why Does the Saltwater Circuit Work? — An ion is an atom that has an electrical charge, either positive or negative. Salt molecules are made of sodium and chlorine. When salt enters water, the water causes the salt's sodium and chloride atoms to pull apart and make the salt crystals begin to disappear. As a result, a sodium ion and a chlorine ion are formed. The sodium ion is missing an electron, which gives it a positive change. The chlorine ion has an extra electron, which gives it a negative charge.
When an electric potential is applied, the positively-charged sodium ions are attracted to the negative pole and the negatively-charged chlorine ions are attracted to the positive pole. These ions carry the electricity through water. The essence of the above process is that an "invisible wire" is formed that allows electrons to move from ion to ion across the water.
Developmental Questions (What discussion questions)
- What do you observe when electricity pass through solid salt?
- Which electrolytes here we can found here?
- What happen when water was added to salt?
- What happen when current passes through salt water?
Evaluation (Questions for assessment of the child)
- Why does salt solution conducts electricity?
- What are the ionic compounds?
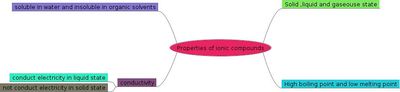
- List the properties of ionic compounds?
Question Corner
To link back to the topic page
Give the link of the page name from where activity was given Back